In Situ Electron Microscopy Reveals How Heat Shapes Stability of Pt Single Atoms on TiO₂ for Photocatalytic Hydrogen Production
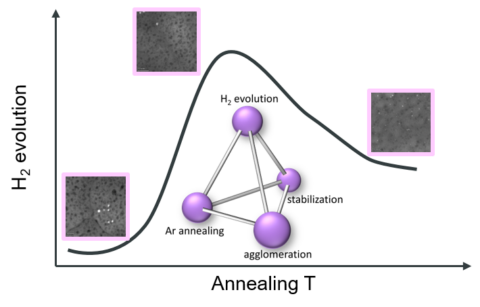
A research team from the Department of Materials Science at FAU has made a significant step forward in understanding how platinum single atoms (Pt SAs) behave under thermal conditions on titanium dioxide (TiO₂) surfaces—crucial knowledge for advancing photocatalytic hydrogen generation. Their findings, recently published in Journal of Materials Chemistry A, highlight how thermally induced agglomeration processes can tailor Pt SAs’ stability.
This study was made possible through the advanced capabilities of the Center for Nanoanalysis and Electron Microscopy (CENEM), and reflects the strong collaboration between the Institute of Micro- and Nanostructure Research (IMN) and the Chair for Surface Science and Corrosion (LKO). Using CENEM’s newly installed in situ gas cell system Climate (DENSsolutions), the researchers captured atomic-scale dynamics of Pt species under inert atmosphere and elevated temperatures. The observations reveal how Pt atoms detach, migrate, and reattach to form clusters, providing insights into the transformation between dispersed atoms and agglomerated species. Complementary ex situ X-ray photoelectron spectroscopy (XPS) and scanning transmission electron microscopy (STEM), alongside photocatalytic performance measurements, enabled the team to identify active sites critical for single-atom photocatalysis. These insights lay the foundation for designing more stable and efficient single-atom catalysts for solar-driven hydrogen production.
For more details:
J. Will., et al., Thermally-induced agglomeration tailors the stability of Pt SAs on TiO2 and use in photocatalytic H2 generation. Journal of Materials Chemistry A (2025) DOI: 10.1039/D5TA00843C